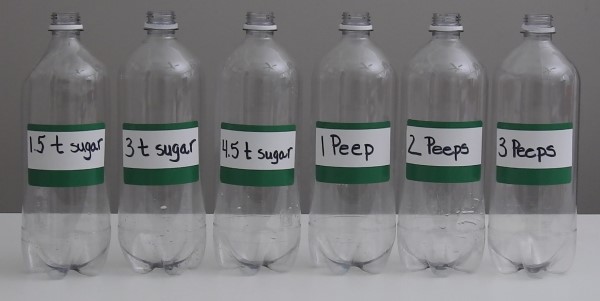Peeps Science Experiment – How to Make a Peep Blow Up a Balloon
Have you ever fermented a Peeps candy and made it blow up a balloon? Peeps science experiment for kids that teaches biology and chemistry. Those cute marshmallowy, sugary candies are the star of this Peeps science experiment.
You may have seen science activities where the adorable little sugary bunnies, chicks, or snowmen are microwaved, put under a flame, or dissolved in alcohol.
In this Peeps science experiment, we are going to make Peeps blow up a balloon! You may have seen the experiment where yeast, warm water, and sugar are used to blow up a balloon.

We are taking that experiment in a slightly different direction. Each Peeps candy contains about 1.5 teaspoons of sugar. So, in this Peeps science experiment, we are investigating several questions:
- Will Peeps candy interact with the yeast and warm water to yield the fermentation process?
- How many Peeps candies do we need to create the fermentation process? Will 1 Peeps candy do it? 2? 3?
- If so, will enough carbon dioxide be produced to blow up a balloon?
- Will the sugar equivalent of 1, 2, and 3 Peeps candies produce the same results as the 1.5 t sugar, 3 t sugar, and 4.5 t sugar bottles. Results being the approximate same size balloon.
We had a surprise result, which we plan to test again. You’ll see it below in the photos we took throughout the Peeps science experiment. This activity is so packed with biology and chemistry, we are excited to share it with you!
If you haven’t downloaded the fun lesson to go with this experiment, put in your email below, and we’ll send it right out to you.
Here’s a video of our Peeps science experiment:
When you do this Peeps science experiment, make it a day of Peeps science with our Change in Mass experiment. You will also want to check out this list of 120 kitchen chemistry and culinary science resources–it is comprehensive and has something for every age group and interest level.
What You Need to Gather:

- 6 cups of warm water about 110 degrees Fahrenheit or 44-45 degrees Celsius
- 6 packets of regular yeast (not fast-acting)
- Sugar, divided into 1.5 teaspoons, 3 teaspoons, and 4.5 teaspoons
- 1 water pitcher that will hold at least 7 – 8 cups of water
- Cooking thermometer to check the temperature of the water
- Small funnel (We we unable to find one of these at the dollar store. But found a small funnel in the baking section at the grocery store.)
- 6 balloons (We used 9” balloons from the dollar store.)
- 6 1-liter bottles with caps
- 3 small bowls
- Labels and marker
Notes:
- You will have so much fun doing this activity, I highly recommend purchasing 12 to 18 Peeps and extra yeast. Your kids will want to take this experiment further, so have some extras on hand!
- 1 Peep 3 contains about 1.5 teaspoons of sugar.
Procedure for the Peeps Science Experiment
Let’s prep some things first:

- Measure 1.5 tablespoons of sugar into one small bowl.
- Measure 3 tablespoons of sugar into a second bowl.
- Measure 4.6 tablespoons of sugar into a third bowl.
- On the six labels write as follows:
- On the first label write: 1 t sugar
- On the 2nd label write: 3 t sugar
- On the 3rd label write: 4.5 t sugar
- On the 4th label write: 1 Peep
- On the 5th label write: 2 Peeps
- On the 6th label write: 3 Peeps
- Place the labels, one each, on a bottle.
- Bring the 6 cups of water to a temperature of 110-112 degrees F. Check your tap water, it may reach this. If not, measure out about 6.5 cups into a glass bowl and microwave for 30 seconds, check the temperature. Repeat if you need to get the temperature close to 110 degrees. You do not want it hotter than 112 degrees. Pour the water into a plastic pitcher.
Now we’re ready to start our Peeps science experiment:
- Line up the six bottles on a work surface as follows 1 t sugar, 3 t sugar, 4.5 t sugar, 1 Peep, 2 Peeps, 3 Peeps
- Pour 1 cup of very warm water into each bottle. This should be 110 degrees Fahrenheit or about 44 to 45 degrees Celsius.

- Using the funnel, empty 1 envelope of yeast into each bottle.

- Using the funnel, empty the 1 t of sugar into the 1t bottle.

- Using the funnel, empty the 2 t of sugar into the 2t bottle.
- Using the funnel, empty the 3 t of sugar into the 3t bottle.
- In the 1 Peep bottle, squish in 1 Peeps candy. You might want to cut up each Peep into smaller pieces before trying to put it into the bottle.
- In the 2 Peeps bottle, squish in 2 Peeps candies.
- In the 3 Peeps bottle, squish in 3 Peeps candies.
- Secure the top onto each bottle and shake each bottle. You will have to shake the Peep bottles a bit more to break up the candy.
- Remove the caps and put a balloon on each bottle. Make sure the balloon is completely attached to the b
- Give each bottle a slight whirl again!
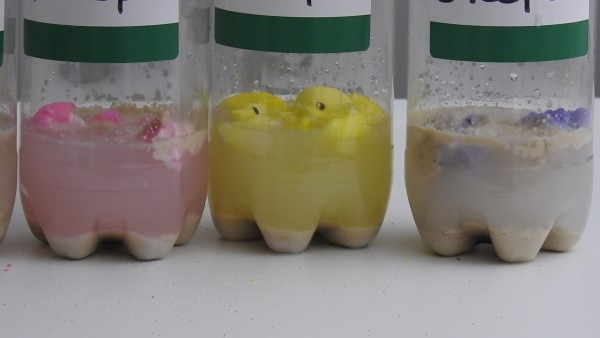
Then let them sit for 30 minutes. Check them often! Do you see the chemical process? (Hint: It’s the bubbles.)


Record your results on the lab sheets we’ve created to go with this experiment.
Let them sit for an hour total? Did any of the balloons get bigger? What happened to the contents of the bottles?
Our Results In This Peep Science Experiment
We had a surprise with our results! We expected the 3-Peeps balloon to get the biggest, but it did not. Was there too much sugar? Was the sugar-yeast-water ratio off? We think this is exactly what happened. In bread baking, it’s important to have use the proper measurements of water, sugar, and yeast. In fermentation, both the yeast and sugar need water. If there is too much sugar, the yeast might be killed or inhibited so much that fermentation cannot take place because the sugar is absorbing all of the water.
We also noticed that our 3t sugar bottle was smaller than its Peep equivalent, the 2 Peeps bottle. 1 Peep = 1.5 teaspoons of sugar, so the 3 t sugar bottle supposedly had the same amount of sugar as the 2 Peeps bottle.
Keep reading, fermentation is explained below…there was a lot of biology and chemistry happening in our Peeps science experiment!
What Was Happening in this Peeps Science Experiment?
What happened in each bottle is a chemical process called fermentation.
But how did this chemical reaction come about? Why did it happen?
For younger students: Yeast is a fungus and uses sugar as food. When the warm water, sugar, and yeast mix, the yeast eats the sugar. During this process the sugar is turned into alcohol and carbon dioxide is created as a result of this process. The process is called fermentation. The carbon dioxide is what fills the balloon in this experiment.
Yeast is used when making bread. Sugar and starch are also in the bread dough along with the yeast. During dough preparation yeast is mixed with sugar and water. fermentation takes place and carbon dioxide gas is produced. This is what makes a slice of bread so soft. Have you ever noticed “air bubbles” in a piece of bread? While the bread is baking, carbon dioxide gas causes the bread to rise as the bubbles push the moist dough up and out.
For older students: Yeast is a eukaryotic, single-celled microorganism. Eukaryotic cells have a nucleus and are found in plants, animals, an fungi. Yeast is actually a fungus that feeds on sugars and starches. So, yeast is a microorganism that, when put in warm water with sugar, eats the sugar (which is also called sucrose) and the by product is alcohol and carbon dioxide. This process is known as fermentation. Ferment is from the Latin word fervere which means “to boil.”
There 1600 species of yeast. Did you know that yeast is related to mushrooms? But the yeast we use in baking is Saccharomyces cerevisiae which means “sugar-eating fungi.”
In fermentation, the microorganism (yeast) when mixed with the warm water and sugar converts the sugar into an alcohol. When the yeast goes through this fermentation process heat and/or a gas is created as a waste product. That gas is carbon dioxide. We may not be able to see the carbon dioxide, but we know the carbon dioxide is present because it fills the balloon.
Yeast is used when making bread. Sugar and starch are also in the bread dough along with the yeast. During dough preparation yeast is mixed with sugar and water. fermentation takes place and carbon dioxide gas is produced. This what makes a slice of bread so soft. Have you ever noticed “air bubbles” in a piece of bread? While the bread is baking, carbon dioxide gas causes the bread to rise as the bubbles push the moist dough up and out.
Get the free lessons that goes with this experiment by requesting it below. We’ll get it right to your inbox!
More Resources:
Check out this BIG kitchen chemistry and culinary science list of resources. Over 110 resources.
A short, to the point explanation of how yeast works in bread baking and some history behind the different types of yeast used in bread making. https://www.finecooking.com/article/the-science-of-baking-with-yeast-2
I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.