Acid and Base Experiment
When studying chemicals, one of the main characteristics scientists look at is the pH level. This determines whether the chemical is an acid, a base, or neutral. Using this knowledge, scientist can predict how the chemical will react with other substances. Use this pH lesson to learn more.
What is pH? What is an Acid and Base Experiment?
What is pH?
The pH level of a chemical refers to the concentration of hydrogen ions in a solution. What is meant by that?
When molecules are put into water, a solution is created. Sometimes these molecules break down in the solution and release a hydrogen (H+) ion. At other times, the molecules will release a hydroxide (OH-) ion. When a hydrogen ion is released, the solution becomes acidic. When a hydroxide ion is released, the solution becomes basic, or alkaline.
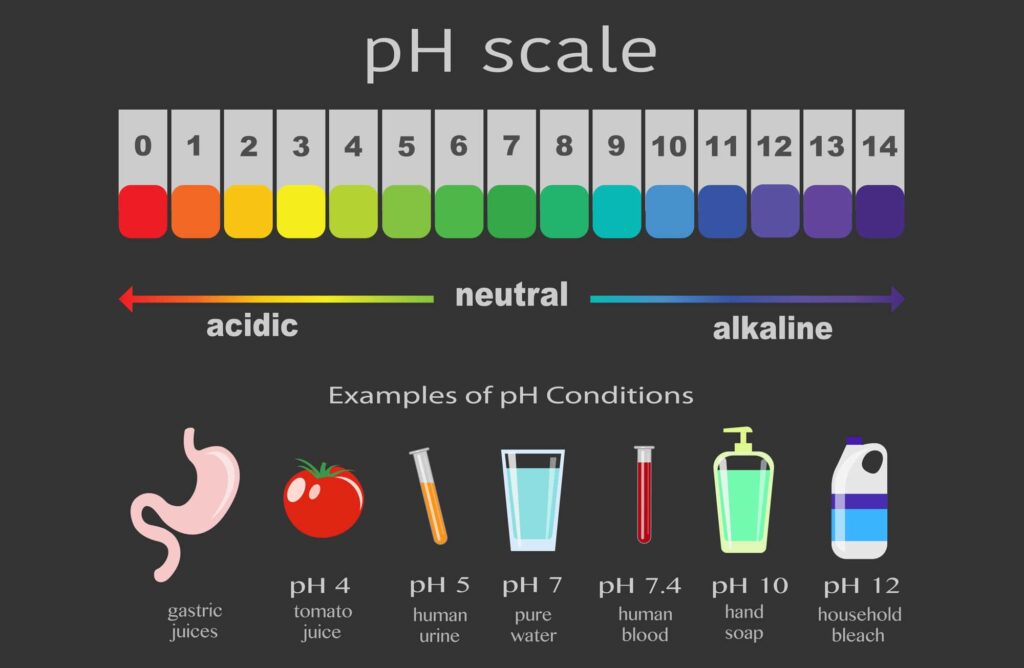
Learn About The pH Scale
The acidity or alkalinity of a solution is measured using the pH scale. This pH scale is used by scientists to measure how acidic or basic a liquid is on a scale from 0 to 14. The lower the pH number on the scale the more acidic a liquid is and the higher the number the more basic. Liquids with a pH of 7 are considered neutral.
The more acidic or basic a solution is the more reactive that solution is with other substances. This means more chemical reactions take place. This is because of the large amounts of hydrogen and hydroxide ions available to react.
Strong acids, such as battery acid, and strong bases, such as sodium hydroxide, are highly reactive and dangerous to handle. These are very corrosive and should be handled in a laboratory or industrial application where great protective measures are taken.
Weak acids, such as acetic acid, and weak bases, such as ammonia, are not as reactive because of the lower amounts of hydrogen and hydroxide ions available, respectively. Even though these are considered acids or bases, they are relatively safe to handle.
How To Measure pH
We have already learned that scientist can use the pH scale to determine the acidity or alkalinity of a solution. But, how do we measure the pH of solutions?
One method to determine pH is to use indicator solutions. These are chemicals that when added to a solution change the color of the solution based upon the pH of that solution. Common indicators are phenolphthalein, methyl orange, methyl red, bromothymol blue, and thymol blue. They each change color at different points on the pH scale. These indicators are all found in one universal indicator solution that can determine the pH level across the entire scale or targeted to a smaller range of the scale. The more targeted, the more accurate the result will be.
Universal indicator solutions can be used in their liquid form and dropped into solutions or universal indicator solutions can be used to treat paper to create pH strips. These strips can be dipped into liquids and the strips will change color according to the pH of the liquid. Comparing the colors to a chart will help determine the pH of the liquid.
Here is a great video about using universal indicator solution by dropping it into a liquid –> https://www.youtube.com/watch?v=GRNslAaXu9k
For a great pH paper experiment, check out the end of this post!
Another method to determine whether a solution is an acid or base is to use litmus paper. These paper strips are treated with special indicator chemicals, but can only tell you if a solution is an acid or base. Blue litmus paper will turn red if the solution is an acid and red litmus paper will turn blue if the solution is a base. Litmus paper does not tell you the strength of the acid or base. You can make your own litmus solution with cabbage. Learn how in this post.
In laboratory settings, a pH meter is used to measure the pH of a liquid. The probes of the pH meter are simply dipped into the liquid and the pH level is given on a digital screen. This is a much more precise measurement of pH than using pH papers. You can get a handheld version for under $20 or a more precise laboratory pH meter for more money. For most student applications, the handheld version is just fine.
Download the Acid & Base Experiment Worksheets
Setting Up An Acid and Base Experiment
Every liquid in your home is either an acid, a base, or neutral. An easy way to find out the pH of liquids you handle everyday is by using pH strips. . Dip the strips into various liquids to see for yourself.
The other day, we were reading an article from LaTrobe University about how brewing a great cup of coffee depends on chemistry and physics. According to the article, one variable that affects the taste of a cup of coffee is the chemistry of the brew water. It was also interesting to read about the physics of the coffee particles!
But, what about the pH level of other substances we use in cooking or might use in cleaning?
This got us testing the pH levels of pantry items such as green tea, vinegar, spaghetti sauce, and more.
Plus, we tested some household cleaning items, like oven cleaner and shower cleaner. (These are highly toxic, so I recommend they be handled wearing gloves and a mask, and sprayed outdoors into a little bowl.) I wanted to use the cleaning items because I knew they would give us a very alkaline (basic) reading.
Design your own pH experiment using other common household liquids and record your results. You might want to try this with orange juice, liquid bleach, different kinds of soft drinks, milk, apple juice, glass cleaner, detergent, and saliva. Before you test them. make some predictions about whether each is an acid, base, or neutral.
Use this pH lesson and pH experiment with your students. Let me know what liquids you tested and if you had any surprises.
Acid and Base Experiment
Materials
- 1 package pH testing paper
- 8 to 12 bowls or containers
- Substances to test – orange juice, lemon juice, tea, coffee, sauces, household cleaners
- 1 pair lab glasses
- 1 pair disposable gloves
- 1 face mask, if testing aerosol cleaners
Instructions
- Determine what substances you are going to test. You might opt for food items.

- You might opt for cleaning solutions. We used these because we wanted to have a very alkaline test result. Please see the precautions listed below.

- Put on eye protection and gloves.
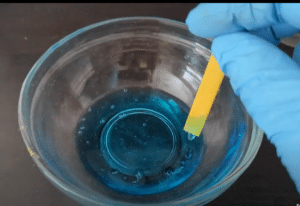
- Place a little of each substance into a small bowl or container. If using aerosol cleaners, go OUTSIDE, wear a mask, eye protection, and gloves before spraying a very small amount into the container. Hold the can very close to the container and away from other people.

- Label each container. You can make labels and place each container with its label.

- Dip one testing strip per container and place it on the surface next to the container. You may opt to put it on a paper towel or other dish to dry.

For more chemistry experiments, lessons and resources…
I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.