Surface Tension In Water Explanation and Experiment

What is Surface Tension in Water?
To understand surface tension in water, we need to look at the structure of water.
A water molecule is made up of two hydrogen atoms and one oxygen atom. There are two ends to a water molecule. The two positively charged hydrogen atoms at one end and the negatively charged oxygen atom at the other end give the water molecules two poles.
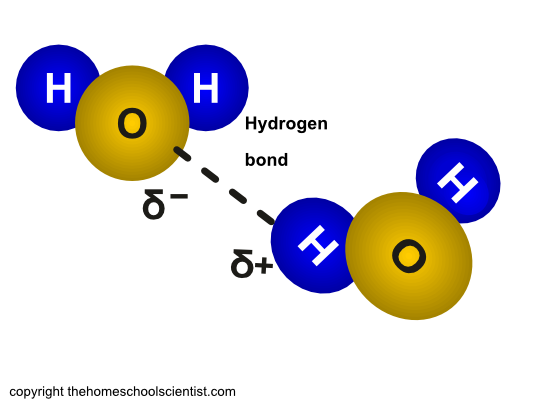
Molecules that are polar will mix with each other. This is called cohesion. This happens in water because the negative charge of the oxygen atom in a water molecule is attracted to the positive charge of the hydrogen atoms in another water molecule.
Cohesion simply means that water molecules like to stick to each other. This is caused by the slightly negative charge of the oxygen atom of one water molecule being attracted to the slightly positive charge of the hydrogen atoms of another water molecule.
When the water molecules stick together they form a “skin”. This skin is strong enough to hold some insects, like water striders, on top of the surface of the water.
Let’s observe surface tension in water with the following activity.
Surface Tension in Water Experiment
Materials
- 1 small bowl of water. Fill about 3/4 full of water
- 1 Sheet of paper towel.
- 1 Small paper clip
Instructions
- Drop the paper clip directly into the water. What happens?
- Thoroughly dry the paper clip.
- Fold the paper towel as shown in the photo.

- Put the paper clip in the center of the folded paper towel.
- Slowly and gently lower the paper towel into the water and wait as it absorbs the water. The paper towel will begin to drop into the water.
- Slowly remove the paper towel up one side of the bowl, and be careful not to touch the paper clip.
- The paper clip will then float!

What Happened In Our Surface Tension in Water Experiment?
Water molecules are polar. This means they have a positive end and a negative end. (You can read more about surface tensions of water, water molecules, and polarity by reading this post on testing the properties of water.
The negative ends stick to the positive ends, and the positive ends stick to the negative ends. This creates a “skin”; we call this surface tension.
When the paper clip was dropped into the water, the “skin” on the water wasn’t strong enough to hold the weight and force of the paper clip.
When the paper towel was used, the weight of the paper clip was evenly distributed on the surface of the water as the paper towel absorbed water and began to sink.
For a more in-depth look at the properties of water, check out our Testing The Properties Of Water post and activities!
MORE WATER EXPERIMENTS
Charcoal Water Purifying Experiment
Learn About The Water Cycle (and an experiment)
I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.



