Testing The Properties Of Water

Testing the Properties of Water
Before testing the properties of water, it’s important for students to know the basic properties of water. The main ones we discuss here are: polarity, surface tension, adhesion, cohesion, and capillary action.
Below is an explanation of 5 properties of water, followed by an easy-to-do activity.
Please note this post may contain affiliate links. Thank you for supporting The Homeschool Scientist.
Table of Contents
Polarity
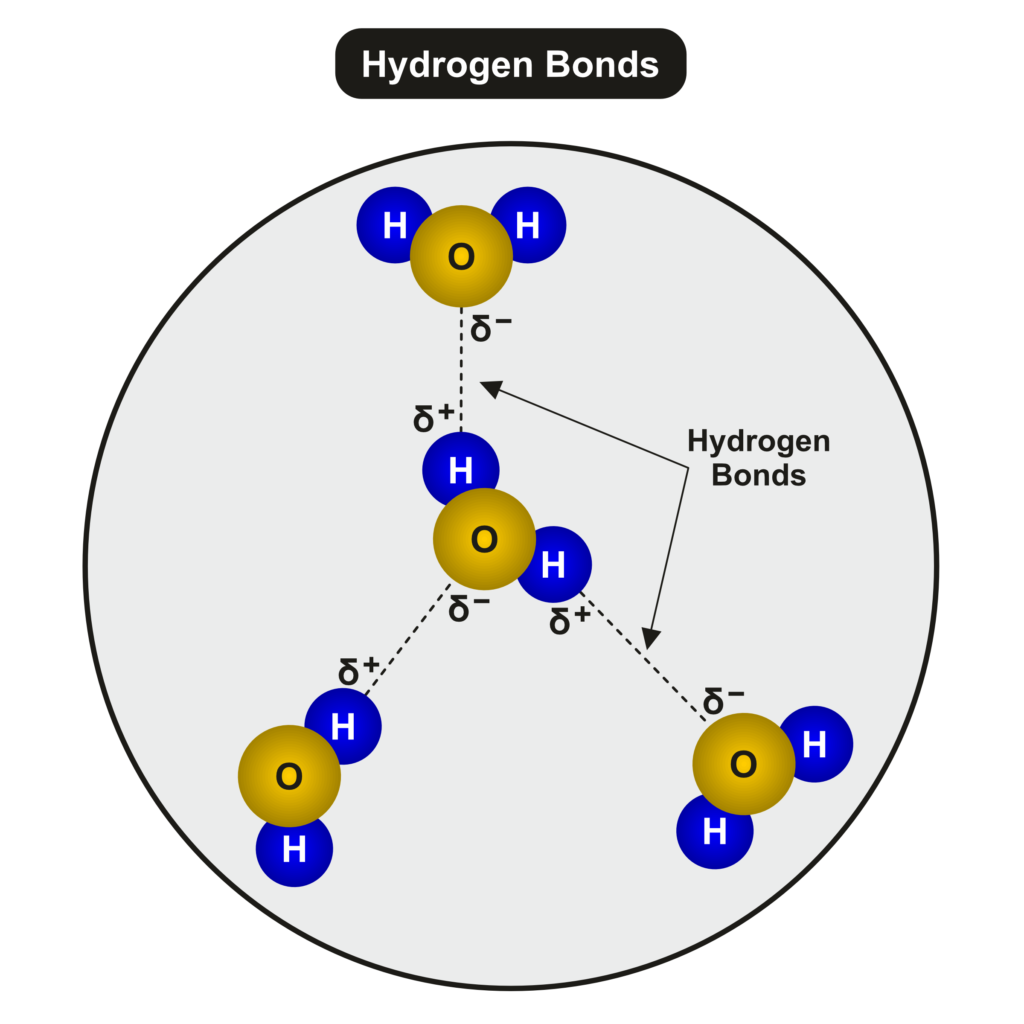
A water molecule is made up of two hydrogen atoms and one oxygen atom. There are two ends to a water molecule. The two positively charged hydrogen atoms at one end and the negatively charged oxygen atom at the other end give the water molecules two poles.
This combination of atoms makes water a polar molecule. Polar molecules have a negative side and a positive side
Pictured above are two water molecules. Have your student identify the two hydrogen atoms and one oxygen atom in each of the two molecules.
Water is also neutrally charged. This means it has an equal number of protons and electrons.
However, even though a water molecule has an equal number of protons and electrons, there is an uneven distribution of the electrons within each water molecule that makes it polar.
So, polarity applies to the distribution of electrical charges around a molecule. In a polar bond, one atom has a partial positive electrical charge, and the other atom has a partial negative electrical charge.
This polarity of water gives it some special properties, like cohesion and adhesion, that can be easily demonstrated and observed in the activities below.
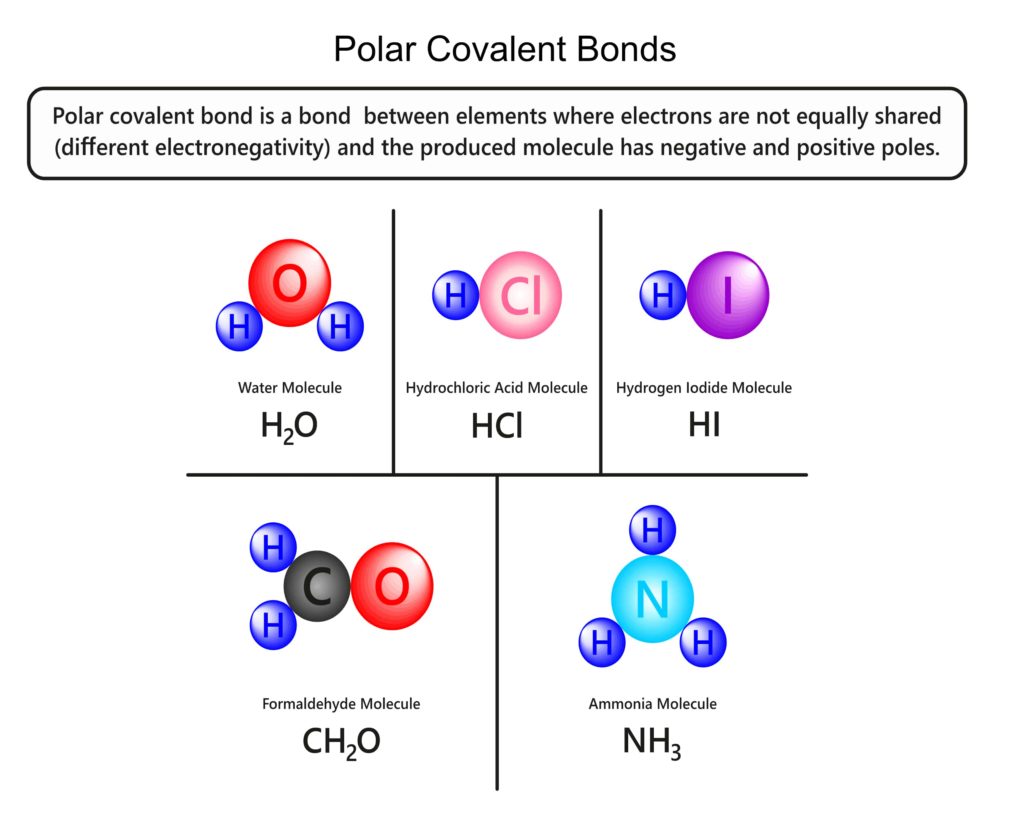
Some examples of other polar molecules are ozone, carbon monoxide, hydrochloric acid, ammonia, and others in the chart below. (This is not the exhaustive list of polar molecules.)
Nonpolarity
Before we move on, let’s talk about nonpolar molecules. Nonpolar molecules have a more even distribution of charge. (Unlike water that is positively charged on one end and negatively charged on the other.)
In a nonpolar bond, atoms share electrons equally, thus there is no partial positive or negative charge between the atoms.
The Behavior Between Polar and Nonpolar Molecules
Polar and nonpolar molecules tend to not be attracted to each other. In other words, polar and nonpolar molecules repel each other.
Polar molecules are attracted to other polar molecules.
Nonpolar molecules tend to be attracted to other nonpolar molecules.
In the oil and water activity below, we’ll be able to see the repelling and attraction of polar and nonpolar molecules.
Cohesion and Adhesion Properties of Water
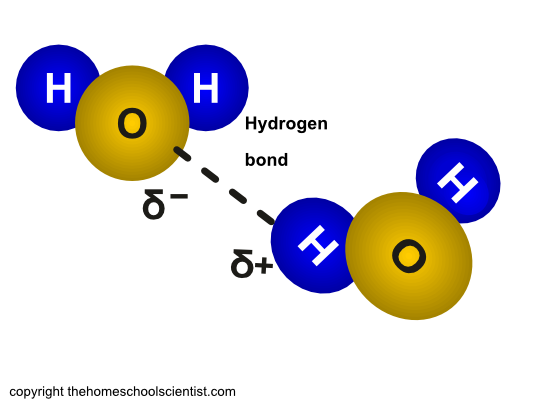
Molecules that are polar will mix with each other. This is called cohesion. This happens in water because the negative charge of the oxygen atom in a water molecule is attracted to the positive charge of the hydrogen atoms in another water molecule.
Cohesion simply means that water molecules like to stick to each other. This is caused by the slightly negative charge of the oxygen atom of one water molecule being attracted to the slightly positive charge of the hydrogen atoms of another water molecule.
The hydrogen bonds between water molecules is illustrated below.
If you have two magnets handy, a great way to demonstrate the concept of a negative end of a water molecule being attracted to a positive end of another water molecule is to hold the north pole of a magnet to the south pole of a magnet. They are attracted to each other and “stick” together.
Related post: Properties Of Liquids Worksheet
Surface Tension
Milk is made primarily of water molecules and water molecules like to stick together. On the surface, where the water meets the air, water molecules cling even more tightly to each other. This causes a “skin” to form on the surface of the water. This skin is so strong that it can hold a weight that normally would sink in water. This is called surface tension.
When the surface tension is disrupted, the heavy object that is floating on the skin will sink. A light object on the surface will be pulled by the attraction of the water molecules if the surface tension is disrupted. This easy experiment will demonstrate that phenomenon.
Cohesion
The surface tension of water is caused by cohesion. Cohesion means that the water molecules like to stick to each other. This is caused by the slightly negative charge of the oxygen atom of one water molecule being attracted to the slightly positive charge of the hydrogen atoms of another water molecule. You can, also, test the cohesion properties of water using an eyedropper, water and a coin.
Capillary Action
The capillary property of water is the ability of water to move through narrow spaces, such as thin tubes or porous materials, against the force of gravity. This behavior is primarily attributed to the combined effects of cohesion and adhesion among water molecules.
As mentioned above, water molecules like to stick together (cohesion), and they also like to stick to other things, like the sides of the straw or the tiny spaces in soil (adhesion).
When water encounters a narrow space, like a thin tube, and the diameter is sufficiently small, the cohesive forces within the liquid, coupled with the adhesive forces between the liquid and the container’s surface, work together. This collaboration overcomes the force of gravity and propels the water upward. It’s like a delicate balance of attractive forces that defies the natural tendency of water to be pulled downward by gravity.
Check out these other activities that demonstrate capillary action:
- Get Growing free printable – 50+ page printable covering plant life cycles, photosynthesis, capillary action, and more.
- Color-changing flowers activity.
- Coffee filter painting
Activities for Testing the Properties of Water
Testing the Properties of Water Activity 1: Surface Tension with Soap and Pepper
A second surface tension activity is listed below.
Testing the Properties of Water Activity 2: Surface Tension – How to Make a Paper Clip Float
You’ll need to gather:
- A paper towel
- A paper clip
- A small bowl filled with water
- Let your student know that they are going to drop a paper clip into the bowl of water.
- What do you think will happen when you drop the paper clip in the water?
- How can we make the paper clip float?
- Now, we’re going to make the paper clip float. Remove the paper clip from the bowl, dry it off.
- Fold the paper towel lengthwise. We used a half-sheet piece of paper towel.
- Place the paper clip on the folded paper towel.
- Hold the paper towel at each end and gently and slowly place it on the surface of the water in the bowl.
- The paper towel will begin to absorb the water and sink into the water.
- Once the paper towel separates from the paper clip, very carefully remove the paper towel from the bowl, leaving the paper clip on the surface.
Explanation of what happened:
Remember, water molecules are polar. This means they have a positive end and a negative end.
The negative ends stick to the positive ends, and the positive ends stick to the negative ends. This creates surface tension.
When the paper clip was dropped into the water, the “skin” on the water wasn’t strong enough to hold the weight and force. When the paper towel was used, the weight of the paper clip was evenly distributed on the surface of the water as the paper towel absorbed water and began to sink.
Testing the Properties of Water Activity 3: Cohesion Will Cost You a Penny!
The surface tension of water is caused by cohesion. Cohesion means that the water molecules like to stick to each other. This is caused by the slightly negative charge of the oxygen atom of one water molecule being attracted to the slightly positive charge of the hydrogen atoms of another water molecule. You can test the cohesion properties of water using an eyedropper, water, and a coin.
You’ll need to gather:
- A coin (penny works well)
- A dropper
- Water

Slowly, drop water onto a coin. Watch as the drops of water stick together to form a larger drop.
Explanation of What Happened:
The water molecules will stick together and form a dome over the coin. Keep adding drops until the drop breaks and spills off of the coin. This is caused by gravity overcoming the force of the cohesion. How many drops of water can you fit on a coin?
Testing the Properties of Water Activity 4: Cohesion in a Glass
What You’ll Need:
- Glass of water filled to the top
- Eyedropper
- Small bowl of water
You can also observe cohesion by filling a glass of water to the top. Next, use a dropper to very carefully add more water until the water is forming an arc of water slightly over the top of the glass. The cohesive properties of the water are holding the molecules together so that they will not spill over the top of the glass. There will come a point when the weak hydrogen bonds can no longer hold and the water will spill over.
Testing the Properties of Water Activity 5: Polarity
In this activity, your student will be able to demonstrate and observe the interaction of polar and nonpolar molecules. We’ll be using water (polar) and vegetable oil (nonpolar).
What you need to gather:
- A plate
- Some water in a measuring cup (a 1/4 cup should do)
- Some vegetable oil (a 1/4 cup should do)
- Food coloring
- Dawn dishwashing liquid
- Mix food coloring in with the water and stir.
- Pour 3 tablespoons of oil onto the middle of the plate.
- Ask your student to observe what happened? Were the drops of oil attracted to each other? Why do you think that happened?
- Next pour the water onto a different section of the plate, but near the oil.
- Pick up each side of the plate slightly off the surface and move the water around the perimeter of the oil. What happened? Are they mixing?
- Knowing what we learned about polar and nonpolar molecules, explain what you are observing.
- Put the plate down on the table and drizzle the Dawn dish soap over the water and oil.
- Pick up the plate carefully on each side and move it around so the soap, water, and oil mingle. What starts to happen? Why do you think this happens?
Explanation of What Happened
The nonpolar molecules of the oil are attracted to each other, so the oil drops come together to form one big “puddle.”
The polar molecules of the water are attracted to each other. But, when the water comes up against the oil, the polar molecules of the water are not attracted to the nonpolar molecules of the oil.
Soap molecules are a bit different. They have an elongated shape. One end is polar and the other is nonpolar. When the soap was added, the polar end of the soap molecule was attracted to the water and the nonpolar end was attracted to the oil.
Now, you can see why soap and water is used to wash dishes and how soap works on grease.
Testing the Properties of Water Activity 6 : Capillary Action
Make a Rainbow with Capillary Action
Materials
- Paper towels
- Box of 4 food coloring bottles
- 4 small jars or clear plastic cups
- 1 white crayon
- 1 water
- 1 baking sheet or foil baking sheet from the dollar store
Instructions
- Fill each jar with equal amounts of water
- Pour 10 drops of red in one jar of water, 10 drops of yellow in another jar of water, 10 drops of blue in another jar of water, and 5 drops of blue and 5 drops of red in the last jar of water.
- Measure the paper towel lengthwise, and draw six (6) evenly spaced vertical lines.

- Draw down each line using the wax stick or white crayon.

- Cut a quarter of the way down each line from step 4. Bend back the second, fourth, and sixth sections.

- Place the jars of water from step 2 on a baking sheet.
- At the same time, place the first section of the paper towel into the red colored water jar, the third section into the yellow water, the fifth section into the blue water, and the seventh section into the purple water.

- Watch as the water travels down the paper towel.

- Once the water has traveled all the way down, remove the paper towel from the jars. Lay the paper towel flat on the cookie sheet to dry.

- Once dry, you'll have a beautiful rainbow, just like the ones after a rain!

What happened?
The water travels through the paper towel for two reasons. First, all paper is made of a sugar molecule call cellulose. Water is highly attracted to cellulose and wants to bond (or stick) to it. Second, the cellulose fibers in a paper towel are made with spaces between them. Since water likes to stick together, the water fills these spaces as it follows the water attracted to the cellulose. More spaces allow more water to be absorbed.
Other Resources Related to the Properties of Water
Make a vocabulary list related to a study of water by using this list of terms and definitions from the USGS.
More Water Experiments
Charcoal Water Purifying Experiment
Learn About The Water Cycle (and an experiment)
Take the USGS Water Properties quiz!
I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.